Digital Mammography System MY-D032E
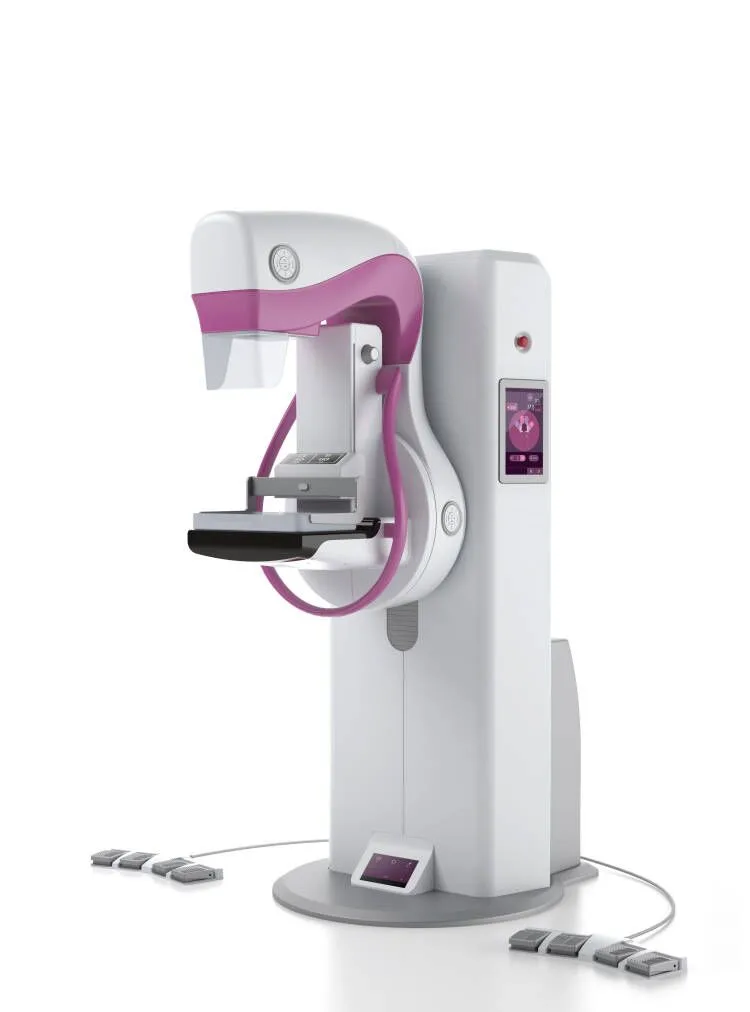
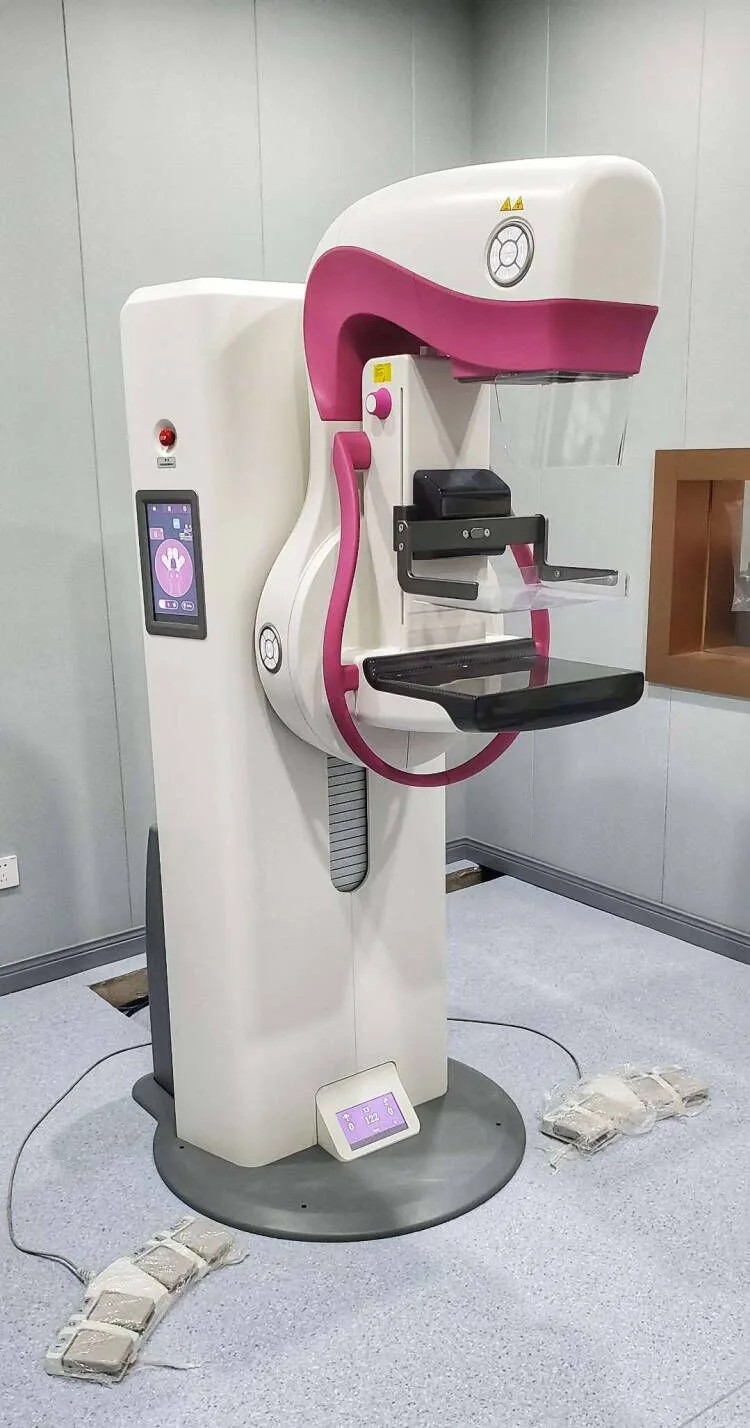
The MY-D032E is a state-of-the-art full-field digital mammography system designed for precision breast imaging and early cancer detection. Equipped with advanced tomosynthesis capabilities and a high-resolution amorphous silicon detector, it delivers exceptional image quality while ensuring patient comfort through intelligent compression technology. Fully compliant with DICOM 3.0 standards, this system streamlines workflow integration and diagnostic accuracy.
Key Features
- Tomosynthesis-ready with software upgradability
- 6 lp/mm spatial resolution for microcalcification detection
- Smart multi-level compression (0-200N force control)
- Dual-side touchscreen control panels
- DICOM 3.0 compatible workstation integration
- Low-dose imaging with automatic filter switching (Rh/Ag)
Technical Specifications
| System Overview |
| Detector Type |
Amorphous Silicon + CsI (24×30cm active area) |
| Pixel Size |
85 µm (2816×3584 matrix) |
| Spatial Resolution |
6 lp/mm |
| Magnification |
1.5× standard (optional 2D biopsy mode) |
| DICOM Compliance |
DICOM 3.0 Print/Store/Query |
| X-Ray System |
| kV Range |
20-40 kV (1 kV steps) |
| Focal Spot |
0.1 mm (small)/0.3 mm (large) |
| Anode Material |
Tungsten |
| Filtration |
Automatic Rh/Ag filter switching |
| Max Power |
11,000 VA |
| Compression System |
| Compression Force |
0-200N programmable |
| Paddle Types |
Standard/Spot/Magnification/Axilla/2D Biopsy |
| Thickness Display |
Dual-side LCD (2-300mm range) |
| Control |
4-foot switch with micro-adjustment |
| Workstation Configuration |
| Acquisition Station |
Intel 2.4GHz CPU, 4GB RAM, 1TB HDD |
| Displays |
1MP (acquisition) + 5MP (diagnostic review) |
| Software Features |
Auto Exposure Control, Image Enhancement, DICOM Networking |
| Certifications |
IEC 60601-1, CE, FDA 510(k) |
| Physical Specifications |
| C-arm Rotation |
±180° isocentric movement |
| Height Adjustment |
650-1350mm motorized column |
| Net Weight |
300 kg |
| Power Requirements |
230V AC, 50/60Hz |